GAP was mentioned for our partnering with the European Prevention of Alzheimer’s Dementia (EPAD) to bring diversity and more volunteers to clinical trials.
Recruiting asymptomatic participants for secondary prevention trials such as AHEAD 3-45 is no small feat. How do you find people who are well on the road to Alzheimer’s disease but feel fine, thank you very much? Scientists cut their teeth on this quest with the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) study, an ongoing secondary prevention trial that took 3.5 years, from 2014 to 2017, to enroll its 1,150 amyloid-positive but cognitively normal participants. At the time, the means were public outreach through a website, advertisements, public appearances by its leaders, etc. Other trialists hosted swabbing parties for ApoE genotyping (Dec 2017 conference news).
In an effort to queue a large group of eager trial participants with known cognitive and biomarker characteristics, scientists led by Paul Aisen at the University of Southern California Alzheimer’s Therapeutic Research Institute, La Jolla, created the trial-ready cohort for preclinical and prodromal Alzheimer’s Disease, aka TRC-PAD, platform in 2017.
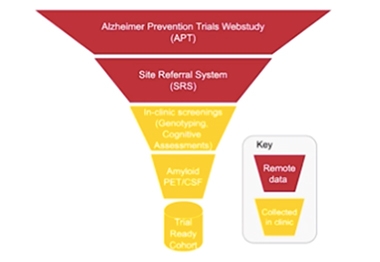
Think of TRC-PAD as a funnel, whereby thousands of prospective study participants join an online registry at the top, and become progressively more characterized—for cognition, genetics, and biomarkers—as they filter their way to the bottom. The resulting amyloid-positive cohort is primed to enter secondary prevention trials.
In a symposium at this year’s virtual Clinical Trials on Alzheimer’s Disease (CTAD) conference, held November 4-7, Aisen and three other leaders of TRC-PAD talked about the platform’s evolution. After three years in the making, it is finally enrolling “trial-ready” participants, and AHEAD 3-45 is the first study to reap the benefits. In August, the investigators published four papers detailing TRC-PAD’s intricacies in the Journal of Prevention of Alzheimer’s Disease.
At CTAD, Aisen said that the first of TRC-PAD’s three major phases is an online web registry called the Alzheimer’s Prevention Trials (APT) Webstudy. It came online in January 2018. Participants answer questions about their family history and any known genotype or amyloid status. Importantly, they also rate their own cognition with the cognitive function index (CFI), and take the Cogstate brief battery test, every three months. An algorithm tracking their performance, and change over time, selects participants likely to have brain amyloid and invites them via a site referral system (SRS) to schedule their first in-clinic visit.
At that visit, participants take more extensive cognitive tests and are genotyped for ApoE. Those deemed at highest risk of having amyloid deposition are referred for amyloid screening. If they are positive, they can move into the trial-ready cohort and await placement into a treatment trial. Researchers used data from the A4 trial to hone the algorithms that move participants from the top to the bottom of the funnel.
So far, the APT Webstudy has 38,000 participants. Alas, roughly two-thirds of them come from established feeder registries, USC’s Sarah Walter reported at CTAD. These include the Alzheimer’s Prevention Registry (APR) at Banner Alzheimer’s Institute, which has supplied nearly 13,000 registrants from its own 352,000 members, the Alzheimer’s Association’s TrialMatch registry, from which nearly 9,000 have joined, UC Irvine’s Consent-2-Contact registry, and UC San Francisco’s Brain Health Registry. In addition to registries, TRC-PAD has been recruiting through social media, local television and newspaper ads, and through study sites. So far, TRC-PAD has been slow to bring in new people “from the wild” (Aug 2016 conference news), a stated goal of large efforts around the globe to find participants for preclinical Alzheimer’s treatment trials.
Unsurprisingly, then, the demographics of APT Webstudy registrants reflect those of its feeder registries: predominantly white and highly educated. Efforts are underway to boost the cohort’s diversity, for example by way of a recently launched Spanish-language version of the online registry, and by reaching out to African American communities, Walter said.
Retention in registries has proved to be another big problem in realizing the vision of large trial-ready cohorts. Partly that is due to people tiring of repeated cognitive assessment, but partly also to thousands of registrants being suspended for years in an online holding pattern while intervention trials are slow in coming. With the APT Webstudy, 96 percent of new registrants completed their initial cognitive screening, but participation dwindled thereafter, with only 38 and 30 percent returning for their next two follow-up assessments, respectively. Only 70 or so loyal APT Webstudy enthusiasts have completed 11 online assessments, Walter said.
TRC-PAD leaders also noted that so far, the Cogstate battery they use to track cognition does not work on smartphones. Getting a phone-friendly test up and running that is similarly sensitive to amyloid elevation is a top priority, Aisen said.
Why is the platform so slow in coming? It took considerable time and effort to gather data and develop algorithms to select and move participants from the web study to the site-referral system, which would involve in-clinic visits. “Just about the week we figured it out, in February 2020, COVID-19 hit,” Sperling told Alzforum. “The timing was disastrous.” The pandemic threw a wrench into TRC-PAD’s progression, as sites shut down observational studies. However, now that pandemic protocols are in place, in-person visits are resuming, albeit at a slower pace than planned.
At CTAD, Gustavo Jimenez-Maggiora, USC, presented current TRC-PAD stats. As of September 2020, 2,450 participants have been referred from the APT Webstudy to a study site; another 342 were referred to study sites from elsewhere. One hundred and fifty have completed an in-person visit, of which 117 have been authorized for amyloid screening. Of the 88 participants who were screened for amyloid so far, 51 were found to have elevated levels. Of those, 36 have enrolled in the trial-ready cohort.
This translates into one amyloid-positive participant for every 1.7 people scanned, Oliver Langford of USC reported in his presentation. This is better than A4, which needed to scan 2.5 people to yield one with amyloid. Still, the funneling process has to speed up considerably to support recruiting for the AHEAD 3-45 and other trials.
Another trial testing CT1812, aka Elayta, a small molecule that shields synapses from A? oligomers, plans to draw from TRC-PAD starting next year. While both these studies are run by the Alzheimer’s Clinical Trials Consortium (ACTC), Aisen told Alzforum that TRC-PAD enrollees will be eligible to join any trial being conducted at their site.
TRC-PAD is working to incorporate plasma biomarkers to improve the screening process. In particular, the researchers plan to incorporate plasma A?42/40 ratios in their screening pipeline. TRC-PAD investigators are currently comparing different A?42/40 plasma assays for their ability to predict amyloid status in the first few hundred participants to undergo amyloid-PET scans or CSF draws. Once an assay is selected, the researchers expect plasma tests will further cut down the number of scans needed to find amyloid-positive participants.
Besides readying a cohort for trials, figuring out how to employ plasma biomarkers for trial screening could prove to be one of TRC-PAD’s most valuable contributions to AD clinical research, Sperling said. The scientists will evaluate plasma and PET side-by-side for now. They are hoping that a blood test deployed early on will further cut down the number of needed, and more expensive, scans, per enrollee.
While TRC-PAD is gearing up, another massive effort to recruit participants for AD clinical trials is shutting down. The European Prevention of Alzheimer’s Dementia study has run out of money (see clinicaltrials.gov). EPAD had been supported for five years by the Innovative Medicines Initiative (IMI), which is funded jointly by the European Union and the European pharmaceutical industry (Aug 2016 conference news).
EPAD aimed to recruit, and deeply phenotype, thousands of potential clinical trial participants across Europe, and then to run side-by-side Phase 2 trials with a shared placebo group and a Bayesian adaptive design. By the time the five-year funding period ended, the cohort had more than 2,000 participants, each phenotyped for multiple cognitive measures, genetics, and CSF biomarkers, but, alas, no drug sponsors to run trials. “We had everything but a drug,” said Craig Ritchie of the University of Edinburgh, who headed EPAD.
Why did no would-be trial sponsor take the plunge? Failed Phase 1 trials were one reason, lack of internal funding another, plus companies hesitated to be the first to take a risk on this innovative platform design, Ritchie said. That there was no funding to maintain the trial-ready cohort as trials took off may have also given sponsors cold feet.
After the loss of IMI funding at the end of 2019, EPAD secured some funds from philanthropic sources to wrap up final study visits and to maintain the massive dataset for the cohort. Then COVID-19 hit, and follow-up visits were halted, too.
But all is not lost. The more than 2,000 participants enrolled in EPAD are “desperately keen” to join a clinical trial, Ritchie said. Around 300 of them are cognitively normal but have amyloid, making them perfect subjects for secondary prevention trials. To help them join trials, Ritchie said EPAD is partnering with organizations such as the Global Alzheimer’s Platform Network (GAP-Net), a U.S.-centered network of study sites that aims to boost the efficiency and quality of AD clinical trials (Mar 2019 conference news). GAP-Net coordinates with TRC-PAD. And of course, participants will be eligible to join trials conducted within their own countries.
While EPAD will not realize its dream of running head-to-head proof-of-concept trials with multiple drugs, Richie hopes that the cohort will nevertheless find its way into clinical trials. Last but not least, the rich EPAD dataset is available to the neurodegenerative disease research community for analysis. (See Parts 1 and 2 of this story.)—Jessica Shugart
Originally posted by AlzForum on November 17, 2020.
