Experts voice concerns over pre-judging promising treatments in the pipeline
Today, five top Alzheimer’s clinicians and researchers called into question the preliminary proposal from the Centers for Medicare and Medicaid Services (CMS) to limit coverage FDA-approved monoclonal antibodies that target amyloid for the treatment of Alzheimer’s disease. The CMS proposal would alter the course of development for the entire class of current and future Alzheimer’s treatments that target amyloid plaques.
“The CMS proposal is a trojan horse that purports to impact one medication but actually will undermine an entire class of treatments for Alzheimer’s,” said John Dwyer, President of the Global Alzheimer’s Platform Foundation®. “This egregious proposal has no statutory basis, no grounding in science or fact, and no legal precedent.”
A letter from the clinicians and researchers urges CMS to change course on their recent proposal, stating: “we have significant concerns about this proposed coverage decision, which would deny or delay patient access to promising new therapeutics based upon: (1) a misunderstanding of the state of the science, (2) requirements to gather evidence in a manner that duplicates the goals of clinical trials required for FDA approval, and (3) a prejudging of evidence that is yet to come.”
The experts go on to say: “In its proposed NCD memo, CMS expresses concerns that clearance of beta amyloid (A?) has not been shown to translate into meaningful clinical improvements in patients with AD.”
CMS has based a large part of its proposal on doubting the science accepted by clinicians and scientists with decades of experience in Alzheimer’s research and patient care. CMS does not have a single neurologist on staff.
“It is critically important to consider that the CMS proposal is based on uninformed doubt on the beta-amyloid cascade,” said Dwyer.
CMS is not the authoritative regulatory body on drug safety and efficacy, and the agency has zero experience evaluating the findings of clinical trials. The agency’s proposed coverage decision undermines the U.S. Food and Drug Administration’s (FDA) expertise and approval of Aduhelm for the treatment of Alzheimer’s disease using its accelerated approval pathway. This sets a dangerous precedent for future drugs approved via the program. CMS is overstepping and using outdated science to defend a decision that is at best discriminatory and at worst illegal.
The experts write “CMS needs to revise the proposed coverage plan in ways that would expand knowledge of the real-world benefits and risks of mABs and allow patients and their doctors to make informed choices.”
The letter was signed by Paul Aisen, Director, Alzheimer’s Therapeutic Research Institute, University of Southern California; Jeffrey Cummings, MD, ScD, University of Nevada, Las Vegas; Marwan N. Sabbagh, Barrow Neurological Institute, Dignity Health/St Joseph’s Hospital and Medical Center; Howard Fillit, MD, The Alzheimer’s Drug Discovery Foundation, The Icahn School of Medicine at Mount Sinai;and Dennis Selkoe, MD, Harvard Medical School and Brigham and Women’s Hospital.
The full text of the comment letter is below.
Comments on CMS Proposed Decision Memo (CAG-00460N) on National Coverage Determination for Monoclonal Antibodies Directed Against Amyloid for the Treatment of Alzheimer’s Disease
PREPARED AND RESPECTFULLY SUBMITTED BY:
Drs. Paul Aisen, Jeffrey Cummings, Howard Fillit, Marwan Sabbagh, and Dennis Selkoe
FEBRUARY 10, 2022
Dear Secretary Becerra and Administrator Brooks-LaSure,
As clinicians and researchers with decades of experience in Alzheimer’s research and patient care, we are concerned about the Center for Medicare & Medicaid Services’ (CMS) proposed National Coverage Decision (NCD) regarding monoclonal antibodies (mAbs) directed against amyloid for the treatment of Alzheimer’s Disease (AD).
We have dedicated our careers to serving patients with AD, so we are aligned with CMS in the desire to better understand mAbs and to ensure that they are safe, effective, and accessible to all patients whom they may benefit. However, we have significant concerns about this proposed coverage decision, which would deny or delay patient access to promising new therapeutics based upon: (1) a misunderstanding of the state of the science, (2) requirements to gather evidence in a manner that duplicates the goals of clinical trials required for FDA approval, and (3) a prejudging of evidence that is yet to come.
Every day, an estimated 1,000 people progress from mild AD to moderate AD. Because individuals with early-stage disease are most likely to benefit from therapeutic intervention with mAbs — and progression may make patients ineligible for treatment in the future — it is important to address these concerns quickly.
CONCERN 1: THE DRAFT DECISION DENIES ACCESS BASED ON A MISUNDERSTANDING OF THE SCIENCE SURROUNDING BETA AMYLOID. IT IS CRITICAL TO BASE THE FINAL DECISION UPON CURRENT SCIENCE.
In its proposed NCD memo, CMS expresses concerns that clearance of beta amyloid (A?) has not been shown to translate into meaningful clinical improvements in patients with AD. However, CMS’s concern that A? clearance is not reasonably predictive of clinical benefit relies on older studies of drugs that are not equivalent to this new generation of medications. As explained by Dr. Cummings, a cosigner of this letter, in a recent publication in the Journal of Alzheimer’s Prevention (1), nearly all the earlier trials failed because they were: “directed at pre-plaque species of A? and did not decrease amyloid plaques. Plaques have been linked by many observations to cognitive impairment in AD, and the effect of mAbs on plaques meets the standard of “reasonably likely” to predict clinical benefit.” (2,3,4)
In addition to the PRIME and EMERGE trials of aducanumab, two RCTs of other amyloid-reducing antibodies (lecanemab, donanemab) demonstrate clinical benefit. The negative data from ENGAGE may be explained (at least in part) by lower exposure to drug compared to the high-dose arms of PRIME and EMERGE. Much more RCT data on amyloid-reducing antibodies will be available over the next year and a half as Phase 3 trials of gantenerumab, lecanemab and donanemab are completed. CMS also cites concerns regarding the lowering of Ab beyond the issue of clinical benefit. We respectfully disagree with these concerns. The fact that Ab monomers are normally generated (a discovery made by Dr. Selkoe, a cosigner of this letter) is not a reason to conclude that lowering amyloid plaques and oligomers interferes with a critical normal function or could be hazardous. The claim that “Ab protects the brain from
infections [and] repairs leaks in the blood–brain barrier” is not widely validated and is not a reason to avoid clearing Ab.
The genetic evidence ascribing a causal role to amyloid accumulation in AD remains compelling. Each of the mutations causing autosomal dominant AD is directly involved in the accumulation of toxic amyloid species. The near universal occurrence of AD in Down syndrome is caused by overexpression of the amyloid precursor protein (APP) because the APP gene resides on chromosome 21. And, the rare genetic mutation (referred to as the Icelandic mutation) associated with a marked reduction in AD is an APP mutation that reduces the generation of Ab.
The reason that amyloid accumulation in the brain is found at autopsy in many people who did not show evidence of cognitive impairment is that amyloid accumulation precedes symptoms by 10–15 years. An observational study of clinically normal individuals with elevated amyloid in the brain showed that over 80% had developed symptoms of AD after 10 years (Donohue et al., JAMA, 2017).
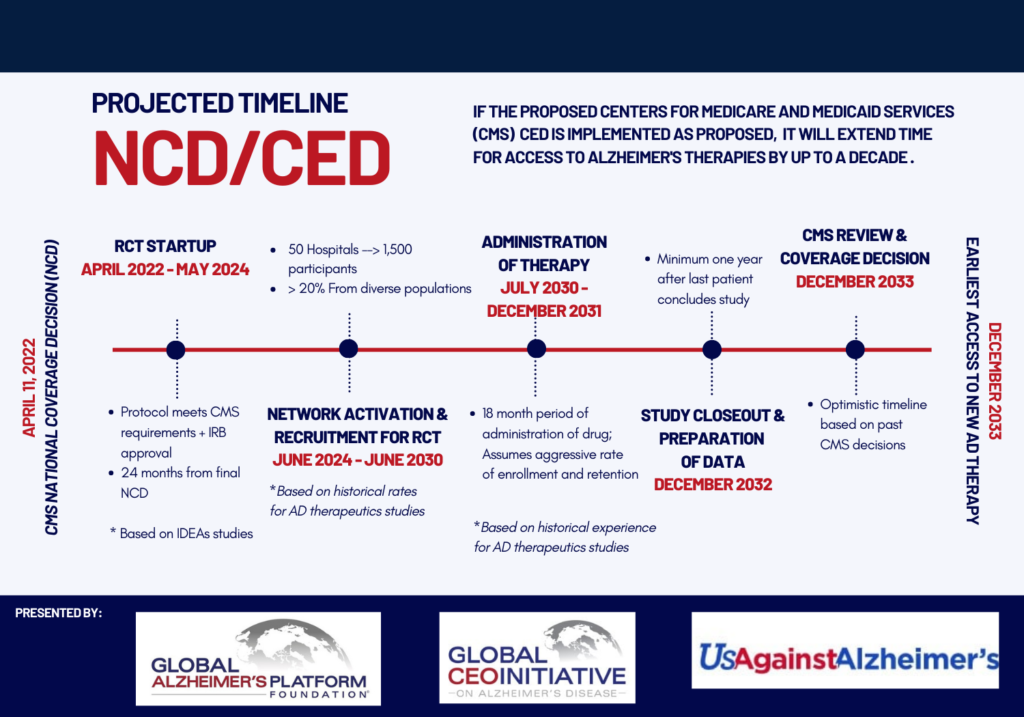
CONCERN 2: THE CED TRIAL REQUIREMENTS AS WRITTEN WOULD CREATE EXCESSIVE DELAYS IN DELIVERING THERAPIES TO PATIENTS WHILE DUPLICATING THE GOALS OF CLINICAL TRIALS REQUIRED FOR FDA APPROVAL AND FAILING TO LEVERAGE EXISTING INFRASTRUCTURE TO EFFICIENTLY GATHER EVIDENCE.
Under the proposed architecture for Coverage with Evidence Development (CED), CMS would require new randomized controlled trials (CMS RCTs) to demonstrate clinical benefit. These proposed CMS RCTs duplicate the goals, work, and patient contribution of clinical trials required for approval by the FDA. We have significant concerns about the viability of executing these CMS RCTs given open questions about who will organize and fund them and about anticipated challenges in recruiting patients into trials. Even if these hurdles are addressed, these CMS RCTs will take several years to conduct, significantly delaying broad access to FDA-approved treatments and exacerbating inequities for communities that cannot access trial sites or are unwilling to participate in research because of concerns related to historical mistreatment of marginalized communities. Large Phase 3 trials of four anti-amyloid antibodies will provide RCT data on the efficacy and safety of such therapies within the next year and a half. Rather than requiring new RCTs, we encourage CMS to consider how patient registries, claims data, electronic health records, and ongoing regulatory trials could be leveraged to develop more-robust evidence on the safety and effectiveness of anti-amyloid immunotherapy.
CONCERN 3: APPLICATION OF THIS DECISION TO AN ENTIRE CLASS OF MEDICATIONS FAILS TO RECOGNIZE DIFFERENCES BETWEEN DRUGS WITHIN THIS CLASS AND PREJUDGES THE RESULTS OF ONGOING TRIALS.
While it is true that the drugs under discussion are all monoclonal antibodies that target amyloid beta in the brain, they are not equivalent drugs. They attack different forms of amyloid beta, and the specific mechanisms of action are different. One monoclonal anti-amyloid antibody, solanezumab, currently in a Phase 3 trial, carries no risk of ARIA. It is not scientifically sound to prejudge the risk–benefit profiles of drugs that are still undergoing development and evaluation based on their membership in a broad class, only one example of which has received FDA approval.
CONCLUSION
Robust data are critical to inform clinical decision-making as well as coverage decisions. CMS should revise the NCD to require workable approaches to evidence development that expand our understanding of the real-world benefits and risks of new mAbs directed against A?. It must base its decisions on an
accurate, up-to-date understanding of the science; must not prejudge the outcome of the scientific process; and must allow patients and their doctors to make informed choices.
We hope to work with CMS to address these concerns, and we willingly offer our input and expertise should you wish to call on us.
Sincerely,
Drs. Paul Aisen, Jeffrey Cummings, Howard Fillit, Marwan Sabbagh, and Dennis Selkoe
Paul Aisen, MD, Professor of Neurology, Director, Alzheimer’s Therapeutic Research Institute, Keck School of Medicine of USC
Jeffrey Cummings, MD, ScD, Research Professor, Department of Brain Health, UNLV School of Integrated Health Sciences; Director, Chambers-Grundy Center for Transformative Neuroscience at UNLV
Howard Fillit, MD, Cofounder and Chief Science Officer, The Alzheimer’s Drug Discovery Foundation; Clinical Professor of Geriatric Medicine and Palliative Care, Medicine and Neuroscience, The Icahn School of Medicine at Mount Sinai
Marwan Noel Sabbagh MD, FAAN, Professor of Neurology, Alzheimer’s and Memory Disorders Division, Barrow Neurological Institute; Clinical Professor of Neurology, Creighton University
Dennis J. Selkoe, MD, The Vincent and Stella Coates Professor of Neurologic Diseases, Harvard Medical School; Co-Director, Center for Neurologic Diseases, Department of Neurology, Brigham and Women’s Hospital
JOINED BY:
1. John Absher, MD, Neurologist, Prisma Health-Upstate
2. Kettia Alusma-Hibbert, DNP, FNP-BC, CNRN, Neuroscience Nurse Practitioner, First Choice Neurology
3. Malgorzata Bach, MD, Neurologist, Northwest Neurology
4. Maryam Beigi, MD, Associate Director of Kagan Clinical Trials at UCLA, UCLA
5. Norman Bettle, MD, Neurologist, Coastal Neurology, PA
6. Mohammad Bolouri, MD, Neurologist, Alzheimer’s Memory Center
7. Adam Boxer, MD, PhD, Endowed Professor in Memory and Aging, University of California, San Francisco
8. Mark Brody, MD, Principal Investigator, Brain Matters Research
9. Megan Casey, RN, Manager of Clinical Research/ Research Nurse, The Memory Clinic, Bennington Vermont
10. Olivia Casey-Moore, BA, Clinical Research Coordinator, The Memory Clinic, Bennington VT
11. Lisa Catapano-Friedman, MD, Medical Director, The Memory Clinic, Bennington, Vermont
12. Bruce Cotugno, MD, Physician, Adult Neurology Center
13. Kirk Daffner, MD, J David and Virginia Professor of Neurology, Harvard Medical School. S Muss Clinical Director, Alzheimer Center, Department of Neurology, Brigham and Women’s Hospital
14. Jose De la Gandara, MD, CPI, DLFAPA, Medical Director, Quantum Laboratories Inc.
15. Guy deBros, PsyD, Clinical Psychologist (Geriatric Neuropsychology), The Memory Clinic
16. Patricio Espinosa, MD, MPH, FAAN, Chief of Neurology, Espinosa Neuroscience Institute
17. Jose Fernandez, MD, Neurology, Hattiesburg Clinic
18. Concetta Forchetti, MD, PhD, Medical Director Memory Disorder Clinic, AMITA HEALTH/ Ascension Chicago
19. Yonas Geda, MD, MSc, Professor, Barrow Neurological Institute
20. Samuel Giles, MD, Neurologist, Memory Treatment Centers, LLC
21. Mark Goldstein, MD, FAAN, Principle Investigator, JEM Research Institute/Headlands Research
22. George Grossberg, MD, Professor & Director, Geriatric Psychiatry, St Louis University School of Medicine
23. Mark Gudesblatt, MD, Medical Director, Comprehensive MS Care Center, South Shore Neurologic Associates
24. Wendell Helveston, MD, Neurologist, Hattiesburg Clinic
25. Lynne Hughes, PhD, Independent Neurology Consultant, LHC
26. Bhupendra Khatri, MD, Neurologist, Center for Neurological Disorders
27. William Klunk, MD, PhD, Distinguished Professor, University of Pittsburgh
28. Elly Lee, MD, Principal Investigator, Irvine Clinical Research
29. Lei Lui, MD, PhD, Assistant Professor of Neurology, Brigham and Women’s Hospital
30. Robert Mannel, MD, Neurologist, Memory Treatment Centers, LLC
31. Paul Mazzeo, MD, Medical Director, Beaufort Memorial Memory Center, Beaufort Memorial Hospital
32. Donald McCarren, DO, FAAN, Neurologist, Memory Treatment Centers, LLC
33. Scott McGinnis, MD, Assistant Professor of Neurology, Harvard Medical School, Brigham and Women’s Hospital
34. Diana Michalczuk, PsyD, Psychologist, Geriatric Neuropsychology, The Memory Clinic, Bennington, VT
35. Richard Mohs, PhD, Chief Science Officer, Global Alzheimer’s Platform Foundation
36. Cynthia Murphy, PsyD, MBA, Executive Director & Neuropsychologist, The Memory Clinic, Bennington, Vermont
37. John O’Bannon, MD, Neurologist, Neurological Associates Inc. Richmond, VA
38. Thomas Obisesan, MD, MPH, Professor of Medicine, Howard University
39. Hamid Okhravi, MD, Associate Professor of Geriatrics, Clinician Scientist, Eastern Virginia Medical School
40. Wally Jamie Plante, MD, Neurologist, Memory Treatment Centers, LLC
41. Anton Porsteinsson, MD, William B. and Sheila Konar Professor of Psychiatry, Neurology, Neuroscience, and Medicine, University of Rochester School of Medicine and Dentistry
42. Aaron Ritter, MD, Clinical Staff, Cleveland Clinic
43. Michael Sauter, MD, Neurologist, Indiana Regional Medical Center, Indiana, PA
44. Douglas Scharre, MD, Professor of Neurology, Ohio State University
45. Ronald Schwartz, MD, Director, Memory Center, Hattiesburg Clinic
46. Rhonna Shatz, DO, Director of Cognitive Disorders Program, University of Cincinnati
47. Amanda Smith, MD, Director of Clinical Research, USF Health Byrd Alzheimer’s Institute
48. Jill Smith, MA, Sr Director for GPS Operational, Global Alzheimer’s Platform Foundation
49. Paul Solomon, PhD, Director, Boston Center for Memory
50. Susan Steen, MD, Neurologist & Clinical Research Investigator, First Choice Neurology/ Axiom Clinical Research
51. Andrew Stern, MD, PhD, Instructor in Neurology, Harvard Medical School, Brigham and Women’s Hospital
52. Robert Stern, PhD, Professor of Neurology, Neurosurgery, and Anatomy & Neurobiology, Boston University School of Medicine
53. Raymond Turner, PhD, MD, Professor, Georgetown University
54. Elizabeth Vassey, PsyD, Executive Director, Boston Center for Memory
55. David Weidman, MD, Neurologist, Banner Alzheimer’s Institute
56. David Weisman, MD, Trialist and Neurologist, Abington Neurological Associates
57. Courtnay Wilson, PsyD, Psychologist, Geriatric Neuropsychology, The Memory Clinic
58. Jeong Hwan Yi, MSW, LCSW, Clinical Social Worker, AMITA Health Neurosciences Institute
59. Kate Zhong, MD, Director of Innovation, University of Nevada, Las Vegas
REFERENCES
1. Cummings, J. Public Policy Should Foster Alzheimer’s Treatment Availability: Comment on the Draft US Medicare Decision to Limit Payment for Aducanumab (AduhelmTM) to Patients Participating in Clinical Trials. J Prev Alz Dis 2022; Published online February 4, 2022, http://dx.doi.org/10.14283/jpad.2022.25
2. Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505-508; DOI:10.1001/ jamaneurol.2013.5847.
3. U.S. Food & Drug Administration. FDA’s Center for Drug Evaluation and Research January 2022. Advancing Health Through Innovation: New Drug Therapy Approvals 2021. 2022.
4. U.S. Food & Drug Administration. Guidance for Industry, Expedited Programs for Serious Conditions – Drugs and Biologics. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER), May 2014.; 2014
STATEMENT OF CONFLICTS OF INTEREST FOR LEAD AUTHORS
Dr. Aisen has received grants from Janssen, NIA, FNIH, Alzheimer’s Association, and Eisai. He also provides consulting for Abbvie, Biogen, Hengrui USA, ImmunoBrain Checkpoint, Merck, Rainbow Medical, Roche, Shionogi, and Vigil Neuroscience, Inc.
Dr. Cummings has provided consultation to AB Science, Acadia, Alkahest, AlphaCognition, ALZPath, Annovis, AriBio, Artery, Avanir, Biogen, Biosplice, Cassava, Cerevel, Clinilabs, Cortexyme, Diadem, EIP Pharma, Eisai, GatehouseBio, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna, Lexeo, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Otsuka, PharmacotrophiX, PRODEO, Prothena, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, Unlearn AI, Vaxxinity, VigilNeuro, Zai Laboratories pharmaceutical, assessment, and investment companies.
Dr. Fillit is the Chairman of the Independent Data and Safety Monitoring Board for Alector, and provides consulting for LifeWorx, Samus Therapeutics, Otsuka and Pineton
Dr. Sabbagh provides consulting for Eli Lilly, Eisai, Biogen and Roche.
Dr. Selkoe is a Director and provides consulting to Prothena Biosciences and has served on an advisory board for Eisai.