Purchase of Axiom Brain Health puts Tampa clinical trial site at center of research to find easier way to detect disease in patients.
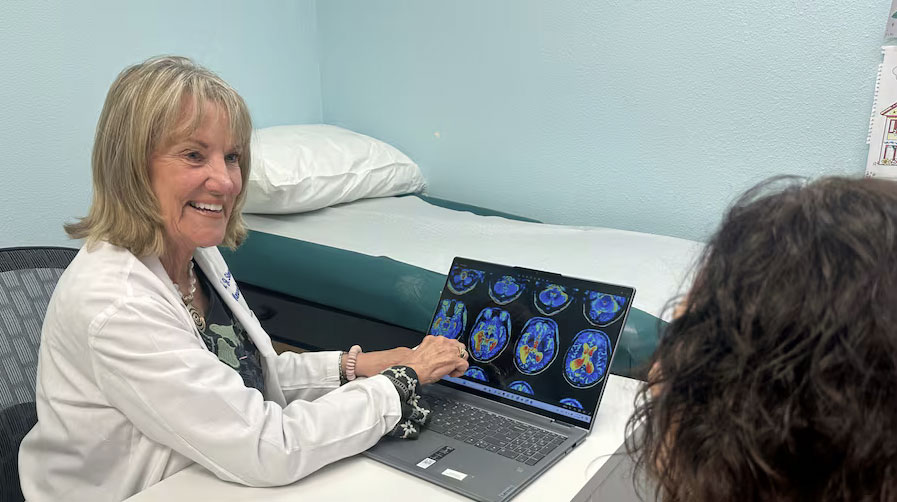
TAMPA — The most reliable way to to diagnose Alzheimer’s disease is through a positron emission tomography or PET scan of the brain, an expensive test not always covered by insurance.
Another option is an examination of cerebrospinal fluid, which requires an invasive and sometimes painful spinal tap.
But a new clinical study is bringing hope that the disease, the most common form of dementia, could in the future be diagnosed with a simple blood test.
Tampa clinical research company Axiom Brain Health was among 13 sites that recruited participants for the Bio-Hermes study of more than 1,000 people across the United States. The research, organized by the Global Alzheimer’s Platform Foundation, revealed how certain blood tests can detect the presence of amyloid in the brain. The clumps of proteins are considered a biomarker, or indicator, of Alzheimer’s disease.
The findings, which were published in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, will make it easier to find patients for future trials for medication and other treatments, said Susan Steen, a neurologist and principal investigator at Axiom.
It could also mean earlier detection of the disease, giving patients a better chance of seeking treatment such as Leqembi, an anti-amyloid drug approved in July by the Food and Drug Administration for the mild dementia stage of Alzheimer’s.
“I think it’s going to be very significant in regard to scientists and researchers and the general population,” Steen said. “It will enable us to have tools to identify and enroll patients more quickly and accurately for clinical trials for Alzheimer’s, which will result in effective treatment we believe.”
Axiom is set to be the lead site for a second Bio-Hermes study with 1,200 participants aged 65 and older. The Tampa firm will also be central to future research after it was purchased in January by the Global Alzheimer’s Platform Foundation, a Washington D.C. nonprofit working to improve research.

The terms of the purchase were not disclosed but Foundation CEO John Dwyer said it was to give the foundation its own clinical research arm. Previous Alzheimer’s trials have suffered from being expensive, long-running with many failing to produce useful results, he said. Some trials suffered from enrolling people who didn’t have Alzheimer’s but other neurological conditions like Parkinson’s
John Dwyer, president and chief executive officer of Global Alzheimer’s Platform Foundation[ Global Alzheimer’s Platform Foundation ]
“We realized we needed a best in class clinical trial site all of our own so we could start doing research and development,” he said. “We wanted to create better clinical trial designs and offer the community a better faster and more accurate process.”
Axiom, led by Steen, will operate as a subsidiary of the foundation. Investment has been made in refurbishing Axiom’s current location with plans in the works for a bigger location and more researchers, Dwyer said.
“We think what we’re doing in Tampa will be a great benefit to the Tampa community,” Dwyer said.
The foundation plans to seek approval from the Food and Drug Administration to use blood tests to prescreen patients with those who test negative for the biomarker avoiding the expense and discomfort of PET scans and spinal taps, Dwyer said.
About 7 million Americans suffer from Alzheimer’s, according to the Alzheimer’s Association. That Centers for Disease Control and Prevention predicts the number of cases will triple by 2060.
![Kaden Appleberry of Axiom Brain Health processes labs for a clinical trial at the Tampa clinic. [ Global Alzheimer’s Platform Foundation ]](https://globalalzplatform.org/wp-content/uploads/2024/04/axiom-staff.jpg)
[ Global Alzheimer’s Platform Foundation ]
Axiom was founded 30 years ago by Steen and other researchers as a way to make cutting edge treatments that could only be offered as part of a clinical trial available to patients. The company also enrolls patients in multiple sclerosis, essential tremor and migraine studies.
The Bio-Hermes study was also a milestone in being one of the first large-scale Alzheimer’s trials to have a participant pool that comes close to matching the population’s demographics. Nearly one-quarter of those who took part were from racial or ethnic groups that have been historically underrepresented, Steen said.
The new trial will seek to emulate or exceed that level of diversity since existing research shows that Alzheimer’s is two times more prevalent among Black people and 1.5 times more prevalent among Hispanic populations.
“We don’t know why,” Steen said. “This data is extremely important in developing drugs and identifying people with this disease.”
This article was originally published at Tampa Bay Times
